The more we know, the more we can help
Research Studies
The Foundation funds research into Smith-Lemli-Opitz and strives to increase awareness about the syndrome.
Studying life with SLO
Natural History Study
Watch the video on the right to listen to Dr. Simona Bianconi, MD discuss the SLOS NIH natural history study.
Be part of progress
Participate in a Study
Researchers at the National Institutes of Health (NIH), in Bethesda, Maryland, are seeking individuals with SLOS (Smith-Lemli-Opitz syndrome) or related cholesterol disorders to participate in a research study. This is a neurodevelopmental disease, with symptoms often apparent in infancy or early childhood. Individuals with SLOS may have symptoms such as developmental delay, challenging behaviors, low muscle tone, failure to feed, poor growth, and sometimes abnormalities in other organs.
The main purpose of this research study is to learn more about the signs and symptoms of SLOS to better understand it and how it develops over time. The NIH also hopes to identify markers of disease to serve as targets for future therapeutic trials.
Who can participate in this study?
- Any person who has been diagnosed with SLOS or related cholesterol disorders such as desmosterolosis, lathosterolosis, CHILD syndrome, X-linked dominant chondrodysplasia type 2, and some cases of Antley-Bixler syndrome
- Healthy (unaffected) family members of individuals with the above disorders.
- Children must have guardian consent to participate.
What is involved?
- All participants will have medical exams and evaluations by experts in the field.
- Blood, urine, spinal fluid, and skin biopsy samples may be collected.
- There is no cost for study-related tests or procedures.
- Lodging and domestic travel compensation will be provided.
- Telehealth visits for those who are unable to travel to the NIH.
Study Name & Link
Study #000487-CH
Natural History Investigation into Biochemical and Phenotypical Aspects of Smith-Lemli-Opitz Syndrome and Related Disorders of Cholesterol Metabolism
Location
National Institutes of Health
Clinical Center
9000 Rockville Pike, Bldg. 10
Bethesda, Maryland, USA
Contact Information
To find out more about this study, please call toll-free:
1-800-411-1222
TTY: 1-866-411-1010
Online: https://clinicaltrials.gov/

Dr. Samar Rahhal
sa**********@*ih.gov
+1-301-793-2122

Other Current NIH Studies
Dear Caregiver of a Child or Adult with Smith-Lemli-Opitz syndrome,
Andrea Bowling is a nurse practitioner working with me at the NIH Clinical Center on the Smith-Lemli-Opitz natural history study. We are reaching out to see if you would be interested in participating in a project that she is leading supporting those providing care for children and adults with a rare disorder.
This will be a virtual study, you do not have to come to the NIH in person.
The project aims to evaluate the impact of three brief group sessions for parents of a child or adult with a rare disease on anxiety and coping. To be eligible, you must be a caregiver of a child or adult with a rare disorder, over 18 years old, and able to understand English.
Participation includes:
- Signing a consent form. A link to the consent form is provided below. If you sign the consent, you will be asked to provide days and times most convenient for you to attend the 3 group sessions.
- Completing three questionnaires prior to the group sessions. The Questionnaires include demographic questions (14 questions), an anxiety questionnaire (the State portion of the State-Trait Inventory (S-STAI), 20 questions), and a coping pattern questionnaire (the Coping Health Inventory for Parents (CHIP) subscale, 18 questions). These will take 15 – 30 minutes to complete.
- Completing three 1-hour group sessions. A follow-up email will be sent to confirm the dates for the session. The group leader will help facilitate conversations and offer skills on recognizing anxiety and improving coping. The facilitator will not have access to your questionnaire answers. Likewise, Ms. Bowling will not have access to the information discussed during the sessions. The group leader will inform Ms. Bowling about the general topics discussed but will not report details about the sessions.
- Completing four questions at the end of each group session. These are asked to help improve the sessions.
- Completing the S-STAI and CHIP subscale after the third session.
Participation is voluntary.
If you have any questions, please do not hesitate to email Andrea Bowling at bo*******@*ih.gov. If you would like to participate, please click here, and you will be routed to the consent form. After reading the consent you will be asked to decide if you would like to participate.
Thank you for your consideration of this study,

Dr. Samar Rahhal, MD
sa**********@*ih.gov
+1-301-793-2122


Participate in a SLOS Research Study
Complete and submit the information requested below.
Someone from our leadership team will connect with you soon.
Thank you for considering being part of a SLOS research study.
Testimonial
What Study Participants Say

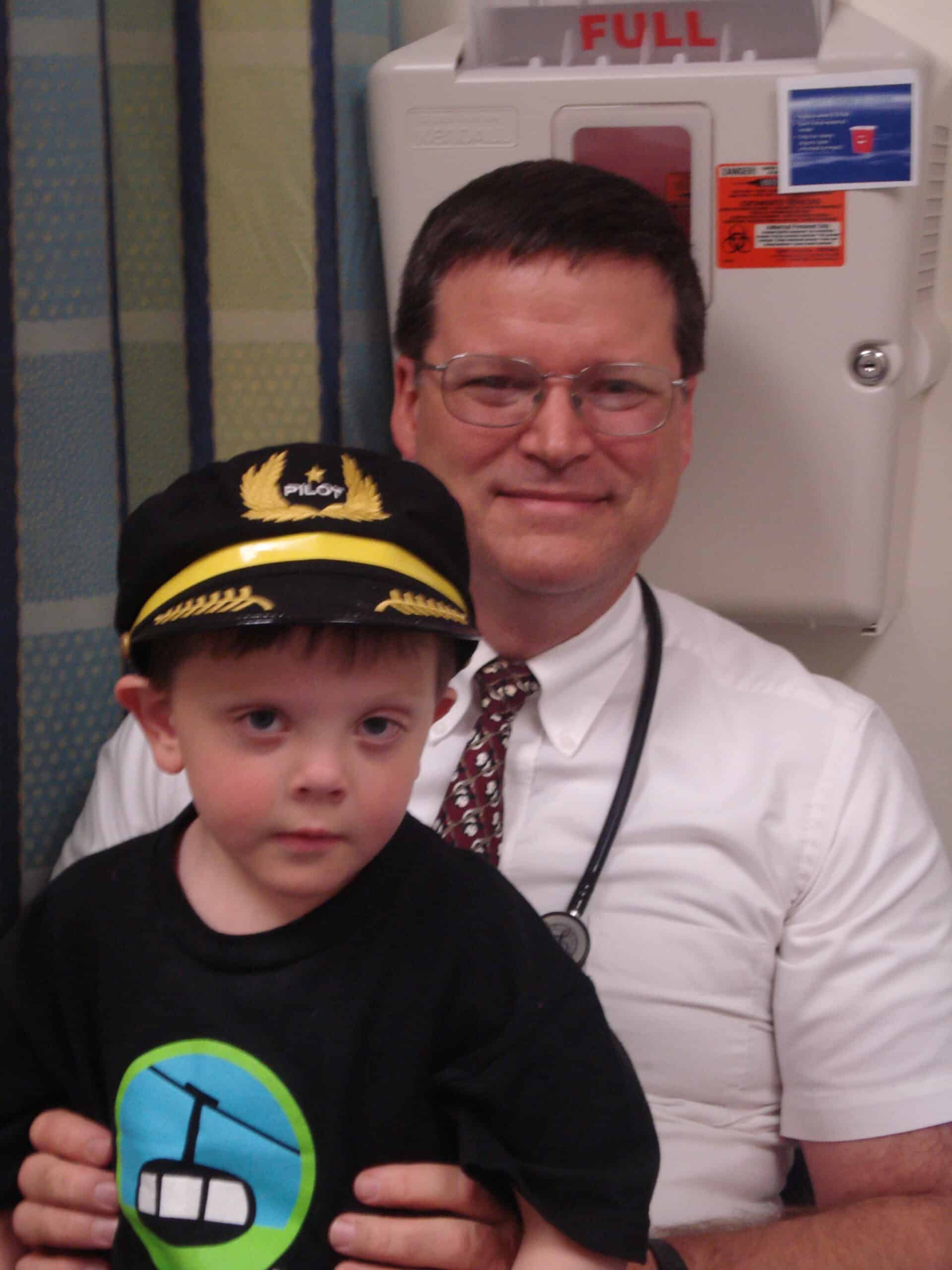
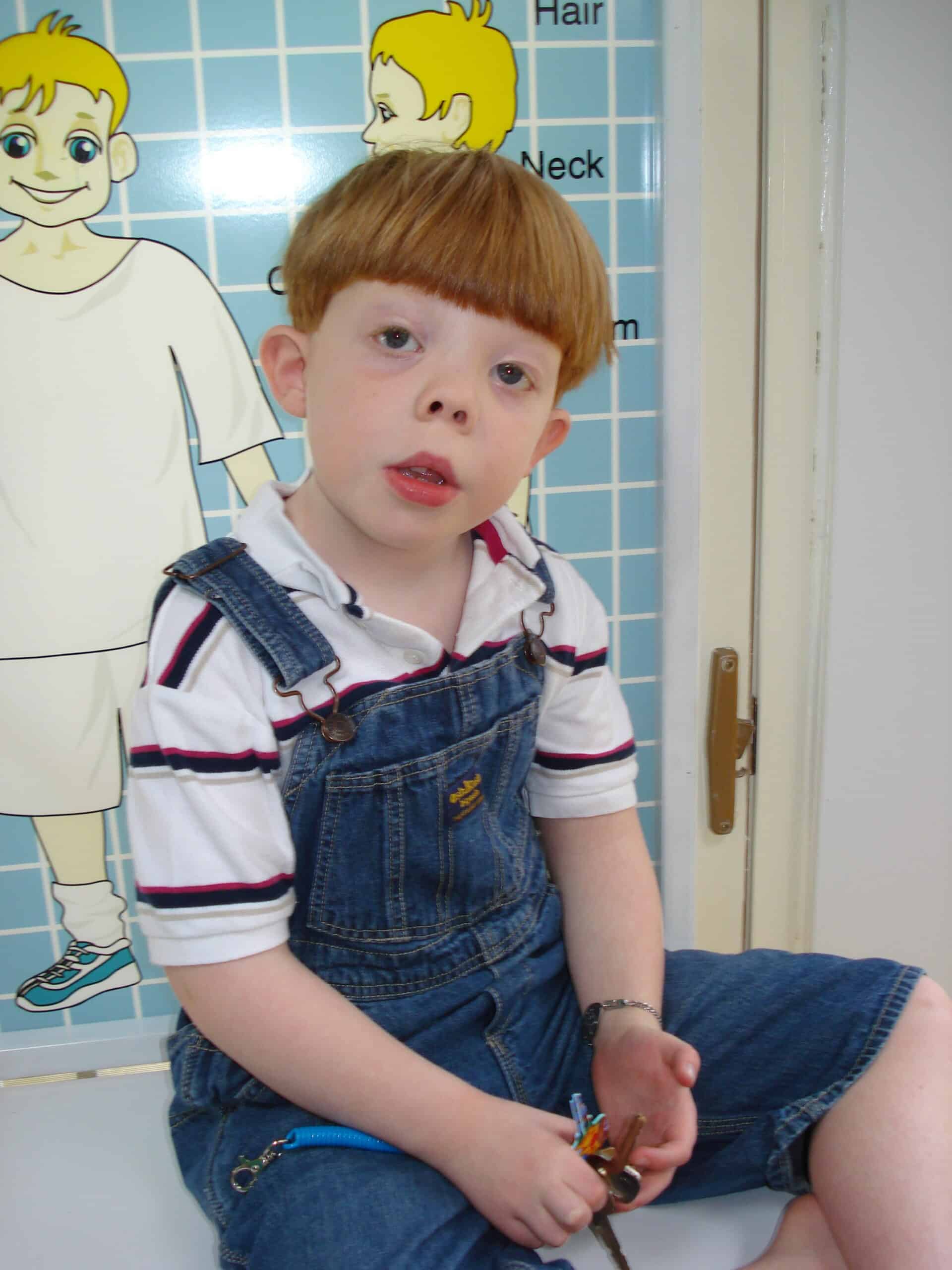
Smith-Lemli-Opitz Foundation Member Family